
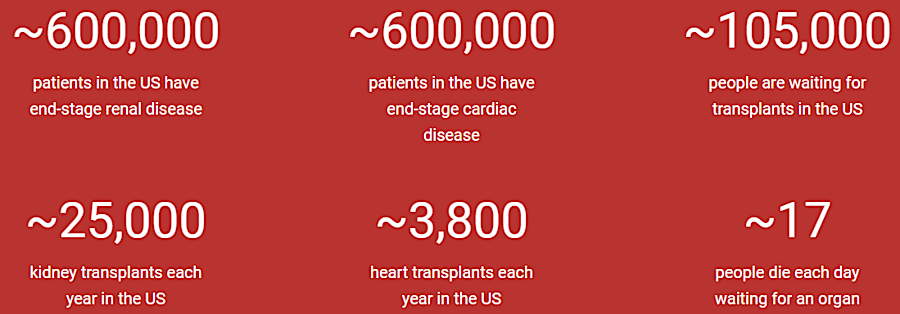
there is a strong demand for organs to be transplanted
Source: Health Resources and Services Administration, Organ Procurement and Transplantation Network Modernization Initiative

there is a strong demand for organs to be transplanted
Source: Health Resources and Services Administration, Organ Procurement and Transplantation Network Modernization Initiative
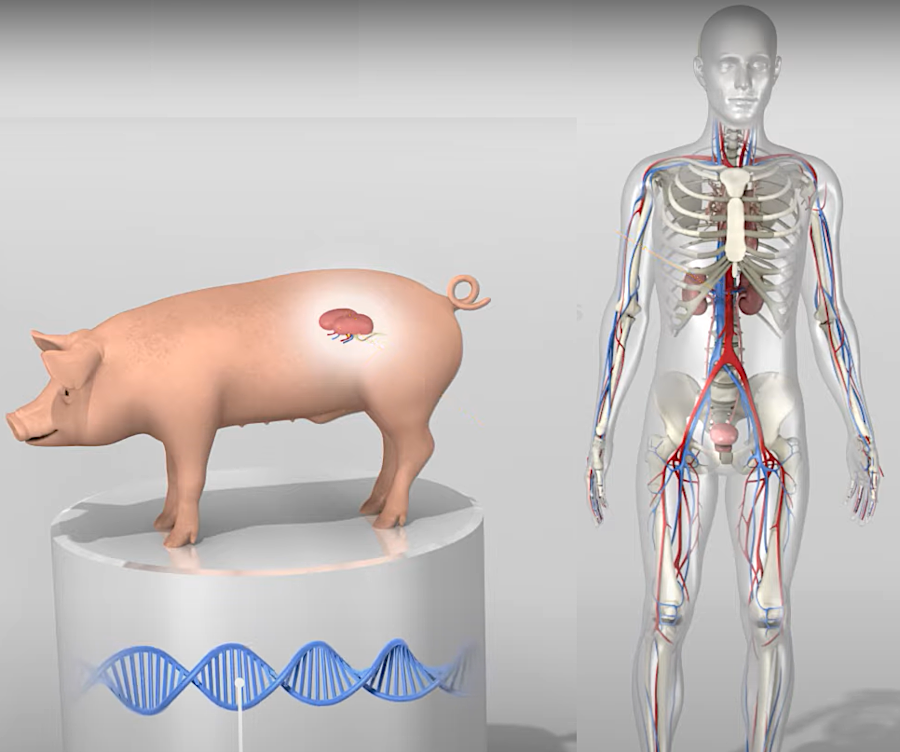
Revivicor, a subsidiary located in Blacksburg of United Therapeutics, is raising genetically engineered pigs for xenotransplantation into human patients. The pig hearts and kidneys will replace diseased and damaged human hearts and kidneys. The company built a $100 million organ production facility in Christiansburg where it could raise 200 pigs, anticipating Federal approval of xenotransplantation procedures. Another facility at Research Triangle Park in North Carolina was closer to medical facilities that conduct transplants on a routine basis, using organs from human donors.
Starting in 2024, Revivicor planned to produce 125 organs per year. There was room for growth, assuming research and then clinical trials succeeded, with a company goal of 3,000 per year. Of the 100,000 people on a transplant list in the United States, 90,000 needed kidneys and about 3,300 needed hearts. Of those waiting for a transplant, 17 died each day.1
In 2022, a human who did not qualify for a traditional heart transplant heart had his removed and replaced with a pig's heart. The first patient lived for 47 days, but then died of heart failure. The patient was severely immunocompromised, and that limited the drugs which could be used to prevent rejection of the foreign tissue.
The heart had porcine cytomegalovirus (PCMV), which was latent at the time of the transplant and was not detected in testing. Since that experiment, more sensitive assays have been developed to detect viruses before selecting a donor pig organ.2
A pig's heart provided by Revivicor was transplanted into a second human in September, 2023. Three pig genes had been removed and six human genes had been inserted into the pig genome, in order to minimize rejection by human tissue.
The xenotransplantation patient was too ill to qualify for any other transplant program. Under the Food and Drug Administration's "compassionate use" policy, experimental procedures are authorized for patients with a life-threatening condition.
Before the surgery the patient said:3
kidneys from genetically-engineered pigs could be transplanted into humans
Source: University of Alabama at Birmingham, Pig-to-human kidney transplant 3D animation
The third transplant of a genetically-engineered kidney from a pig to a human was completed in December, 2024. Of the previous two kidney and two heart xenotransplants, none of the patients had lived more than two months. Healthy baboons with a pig-derived transplant had survived in tests for a year, but the human patients had been near the end of their lives when they received such transplants under the "compassionate use" rules of the Food and Drug Administration.
The third kidney transplant used a kidney with 10 edited genes. Revivicor planned to submit a proposal to the Food and Drug Administration to start clinical trials using that organ in 2025.4
In Carroll and Grayson counties, farmers are considering raising specific pathogen-free (SPF) sheep for biomedical research. They are raised to be free from 54 common livestock illnesses, so each sheep is worth over $3,000 compared to $200 for mutton.
At a 2023 conference organized by the non-profit Blue Ridge Plateau Initiative, speakers proposed that specific pathogen-free sheep could become the source for organs transplanted into humans in 5-10 years. It was already possible to remove sheep tissue from organs in order to create "ghost organs" on which human cells can grow. Cells from a patient in need of a kidney could be grown on a ghost organ within a specific pathogen-free sheep. Because the cells in the replacement organ came from the patient, there would be no problems with rejection when transplanted into the patient.
The concept was visionary in 2023, and no one had committed the $15 million to $20 million in capital that would be required to go into production. Bio-secure buildings would need to be constructed inside which humans would wear biohazard suits around the sheep. Fencing would be needed to ensure the pastures were also bio-secure facilities, excluding other animals that might carry infectious organisms.
Virginia Agriculture Secretary Joseph Guthrie said optimistically:5
The pigs used by Revivicor are genetically modified to remove the gene that triggers alpha-gal syndrome (AGS). That syndrome is manifested after being bitten by a lone star tick (Amblyomma americanum). Consuming red meat triggers an allergic reaction to Alpha-gal (galactose-α-1,3-galactose), a sugar molecule found in most mammals.
GalSafe pigs, safe for human consumption and essential for transplanting pig organs, were developed using animals at a Revivicor facility in northern Roanoke County. Revivicor provides samples to people who have alpha-gel syndrome, but to date no company has found it economical to raise GalSafe pigs for meat. A United Therapeutics spokesperson said:6
the demand for transplants exceeded the supply, and Revivicor sought a new way to increase supply
Source: Revivicor
United Therapeutics, which owned Revivicor, announced in 2023 that it planned to construct an organ production facility in Silver Spring, Maryland. That location was in the I-270 biotechnology corridor near the National Institutes of Health, the primary agency of the United States government responsible for biomedical and public health research.7
The U.S. Food and Drug Administration authorized clinical trials by two companies for xenotransplants of kidneys grown in pigs. The Federal agency gave United Therapeutics permission to start with six patients, and moving through up to 50 before receiving broader approval of the process. The kidneys were affected by 10 edits to the genome. There were six human genes added and four natural pig genes inactivated.
The eGenesis trials started with three initial patients. The eGenesis kidneys had 59 pig genes inactivated plus 10 additional gene edits.8